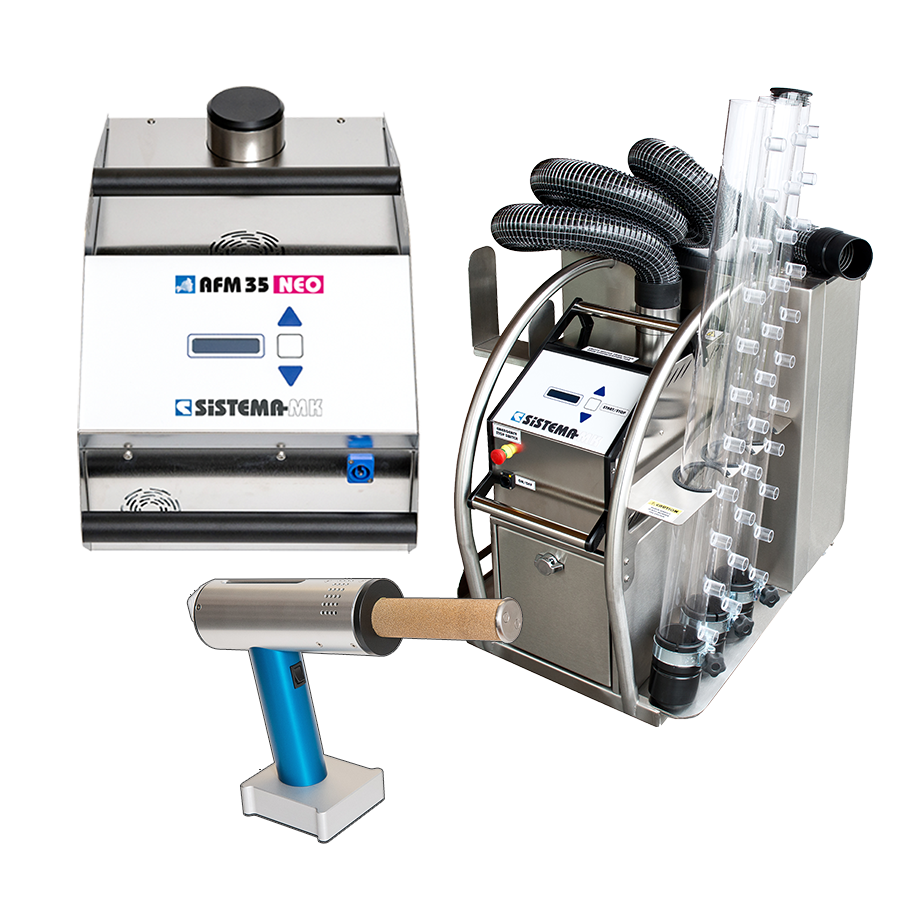
Air Flow Mappers (Clean Room Foggers) AFM series
Sistema-MK makes available three advanced product lines for airflow visualization and cleanroom mapping: the AFM-NEO Series, the Fog Pistol Series, and the apollo (AP) Series, all engineered and manufactured in Germany. The AFM-NEO Series delivers ultrapure, dense, paper-white fog using DI or WFI water, with precise control and high fog output via multiple piezo units. The lightweight, battery-operated Fog Pistol Series provides sterile, residue-free fog for small- to mid-scale applications. For large-scale cleanrooms, the apollo Series generates massive, high-purity fog using nitrogen and ultrapure water. All systems support airflow studies under ISO 14644-3 Annex B7 in critical environments.

AFM-NEO | -DUAL | -CUBE SERIES
Sistema’s AFM-NEO cleanroom fogger series — including the AFM CUBE, AFM24-NEO, AFM35-NEO, AFM40-DUAL — utilizes high-performance, purpose-built piezoelectric modules made from stainless steel, specifically engineered for cleanroom applications.
All models in the AFM-NEO series are designed to operate exclusively with deionized (DI) water or Water for Injection (WFI) as a single fluid medium, ensuring compliance with cleanroom standards and supporting ultra-clean fog generation for critical environments.

NEUTRAL BUOYANT AFM FOG PISTOL
The Fog Pistol Series (AFM Fog Pistol EVO.1 and EVO.2) features a lightweight (only 974–1040 g), compact design. It is battery-operated and equipped with a 60 ml refillable tank that provides approximately 60 minutes of continuous operation. The fog produced is neutrally buoyant, sterile-grade, clean, odorless, and residue-free, with “zero momentum,” making it ideal for small- to mid-scale airflow visualization, leak detection, and similar applications.

apollo AP series nitrogen cleanroom fog machines
The apollo (AP) Series (AP35-4 I AP50-4, AP100-4 and AP150-4) are nitrogen / water-fog machines for very large-scale, high purity cleanroom applications. The systems generate massive fog volumes with dry, paper-white fog, using nitrogen and ultrapure water, with built-in fans, large tanks/dewars, flexible output, and suitable for large ISO cleanroom zones requiring high fog output over extended durations.
Airflow Visualization in Pharmaceutical Cleanrooms: ISO 14644-3 Annex B7 and the Strategic Role of Ultrapure Fogging Technology
Airflow visualization is a central element in the qualification and monitoring of pharmaceutical cleanrooms. Regulatory bodies increasingly expect not only technical compliance but also a strong demonstration of a facility’s overall contamination control strategy. ISO 14644-3 Annex B7 provides the standard framework for airflow visualization, but the choice of technology is strategic: it influences regulatory confidence, audit outcomes, and ultimately the manufacturer’s reputation for quality.
Visualization of airflow from a strategic perspective and advantages of Sistema’s three airflow mapping cleanroom fogging devices in this regard
Pharmaceutical cleanrooms exist to protect patients by preventing microbial and particulate contamination in sterile pharmaceutical products. Airflow visualization is not just a test; it is visual proof of a facility’s contamination control philosophy. ISO 14644-3 Annex B7 requires manufacturers to verify and document that air behaves as intended — unidirectional in Grade A zones and protective at boundaries. Regulators such as the FDA and EMA have made it clear: video recordings of airflow patterns are not optional but expected evidence in audits.
From a strategic standpoint, poor visualization or inadequate documentation can undermine confidence during an inspection. Conversely, clear and well-presented airflow studies strengthen a facility’s compliance posture and demonstrate a culture of quality.
Strategic Purposes of Airflow Visualization
While airflow visualization is a technical exercise, it has broader strategic functions:
- Regulatory Assurance: Convincing inspectors that cleanroom design and operation are effective.
- Risk Management: Identifying and mitigating risks related to airflow disruptions, reducing the chance of costly product recalls.
- Stakeholder Communication: Providing a clear, visual language that bridges engineering, quality assurance, and executive decision-making.
- Operational Excellence: Reinforcing operator training and awareness of contamination control principles.
Seen through this lens, the fogging technology selected is not just a tool but a strategic asset.
Why Using the Right Fogging Technology in Cleanrooms Matters
Traditional fogging systems can produce unclear or short-lived airflow traces, leaving engineers struggling to capture convincing footage. In a regulatory inspection, this can mean the difference between smooth approval and additional scrutiny. Further, any risk of contamination from the fogging agent itself can raise questions from auditors.
Strategically, a cleanroom operator must consider:
- Audit Readiness: Will the visualization videos impress inspectors with clarity and reliability?
- Risk of Contamination: Does the fogging method itself compromise GMP standards?
- Resource Efficiency: Is the process efficient enough to avoid downtime and delays?
These questions make the choice of fogging technology a management-level decision.
Advantages of Sistema’s three airflow mapping cleanroom fogging devices in this regard
Sistema’s three airflow mapping cleanroom fogger series, developed by German manufacturer Sistema, provide ultrapure paper white fog generated exclusively from ultrapure water respectively from a sterile fluid with the Fog Pistol series. This design directly addresses the strategic concerns of pharmaceutical operators.
Strategic advantages include:
- Regulatory Confidence: Produces clear, high-density, neutral buoyant fog for crisp video documentation — a strong asset in inspections.
- Zero Residue Risk: Ultrapure water basis ensures no chemical or particulate contamination, fully aligned with GMP.
- Audit-Ready Documentation: Enables high-quality recordings that can be stored, reviewed, and presented with confidence.
- Operational Efficiency: Compact and ergonomic equipment minimizes disruption during qualification or requalification.
- Reputation for Quality: Demonstrates proactive investment in cutting-edge contamination control.
- Extensive and varied range of accessories: Allows users to fine-tune the device to their individual application profile
By employing Sistema’s airflow mapping cleanroom fogger series, facilities not only meet Annex B7 requirements but also strengthen their inspection readiness narrative.
Conclusion
Airflow visualization, guided by ISO 14644-3 Annex B7, is not only a technical requirement but also a strategic communication tool. Sistema’s three airflow mapping cleanroom fogger series empower pharmaceutical facilities to produce compelling, audit-ready documentation while safeguarding GMP standards. For facility and validation engineers, choosing ultrapure fogging technology strengthens compliance, mitigates risk, and reinforces the company’s reputation for operational excellence and patient safety.
References
ISO 14644-3:2019 – Cleanrooms and associated controlled environments — Part 3: Test methods (also referenced as Annex B7 in older versions)
EU GMP / GMP Annex 1 (Sterile Manufacturing) – Draft & Interpretative Guidance
